 I was born in Karachi, in southern Pakistan, in 1985. I weighed about five pounds. My parents were teaching at the Karachi American school at the time. They knew they wanted to adopt a baby while living there. They got the call to come pick me up, three weeks earlier than expected. As I was early, they were not ready; they came to get me ten hours later.
I was born in Karachi, in southern Pakistan, in 1985. I weighed about five pounds. My parents were teaching at the Karachi American school at the time. They knew they wanted to adopt a baby while living there. They got the call to come pick me up, three weeks earlier than expected. As I was early, they were not ready; they came to get me ten hours later.
I was a quiet baby who met developmental milestones, just weaker than average. It took me six months to weigh 10 pounds.
When I was two, I was diagnosed with nephrotic syndrome, a rare kidney disease. I was put on prednisone and was treated from age two to age eight, until I went into remission. The prednisone masked other symptoms, and coming off the prednisone caused my body to become extremely weak. I had to be in a wheelchair for the next two years. Stairs, and even walking down the hall, were a challenge.
At the beginning of fourth grade, our family moved to Asir, Saudi Arabia, where my parents were still teaching in the American school system. I started fourth grade with a brand-new wheelchair. One of the parents at the school was a neurologist, and after observing me, he didn’t think my diagnosis was correct. He referred us to a neurologist he knew at what is now called New York-Presbyterian (then Columbia Presbyterian) hospital for an evaluation.
In the summer of 1995, right before I turned 10, I spent three days at Columbia Presbyterian, with a rotation of 50 doctors who were focused solely on me and doing multiple tests. On day three, I was given a tensilon injection, and my eyes went from being very droopy to wide open. My doctor told me to get out of the wheelchair and walk down the hall and I did, without exhausting. He said that he was going to diagnose me with Congenital Myasthenic Syndrome and would start me on pyridostigmine (Mestinon) that day.
I no longer needed my wheelchair. I could walk down the halls. I would still tire faster than another normal-bodied ten-year-old but I was better than I was.
Journey to Advocacy
In 2009, when I was in my early 20s, I was referred to Lahey Clinic in Burlington, Massachusetts, which was a site for a clinical trial of 3,4-Diaminopyridine, known as 3,4 DAP. I was accepted into the trial and then received 3,4 DAP through compassionate care use for about a decade.
In the mid-2010s, my access to the drug changed. I was told that the medication is not FDA approved for my diagnosis, CMS – only for LEMS. I’d been receiving the treatment off label previously. I went home, told my mom, and we called my neurologist.
The neurologist said that if insurance denied it, the medication would cost $375,000.
We were at a loss. After multiple denials and about a week of no medication, my doctor went for the appeal. Thankfully, it was approved, but I now annually have to prove to my insurance provider that (a) I still have my diagnosis and (b) I need this medication for my diagnosis.
After I was approved by my insurance company, they covered the out-of-pocket costs, and I was paying $40 a month. I switched jobs in the summer of 2023, and although I have the same insurance, I’ve gone from paying $40 a month, to now paying $500 a month.
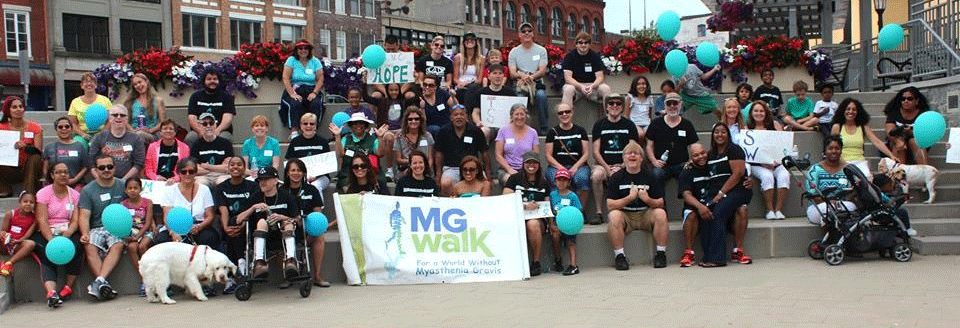
Raising Awareness
Through this journey, I started to raise awareness in my city of New London, Connecticut. I was really starting to feel comfortable with who I was as an adult and being more open about my diagnosis.
In 2015 I held my first myasthenia gravis awareness walk. I made ‘TEAM SHIVVY’ shirts to raise money to buy water and snacks and donated the rest. I held them yearly until Covid happened.
In 2019 I attended my first Rare Disease Week on Capitol Hill with the EveryLife Foundation, in Washington, D.C. The event is part of EveryLife’s Rare Disease Legislative Advocates (RDLA) program. I went again in 2023 and then in February 2024.
Being part of this group has given me a way to channel my experiences – my positive interactions, my frustrations, my hopes and goals – into a powerful movement where I can share my voice. Together we urge policymakers to take action to address the challenges faced by people with rare disease.
Read more about my experience at 2024 Rare Disease Week on Capitol Hill in this blog post.

